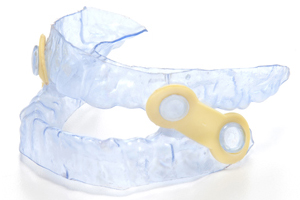
Myerson EMA First Step
FDA Cleared
 Introducing EMA First Step, the new 90 day trial appliance from Myerson for the treatment of snoring and obstructive sleep apnea. EMA’s patented design features elastic straps that allow you to adjust the appliance in one millimeter increments.
Introducing EMA First Step, the new 90 day trial appliance from Myerson for the treatment of snoring and obstructive sleep apnea. EMA’s patented design features elastic straps that allow you to adjust the appliance in one millimeter increments. The elastic straps are latex free and are easily changed allowing free lateral excursion. This device is the perfect way for your patients to discover how they can get a better night’s sleep.
The elastic straps are latex free and are easily changed allowing free lateral excursion. This device is the perfect way for your patients to discover how they can get a better night’s sleep.
If an overnight sleep study confirms successful treatment with an EMA First Step, schedule your patient to receive an EMA custom lab-fabricated final appliance that will last for years.
Make Your Own EMA First Step
Simple and quick in-office fabrication means your patient can leave with an EMA First Step the same day and start sleeping better that night. The EMA First Step Kit includes everything you need and is available exclusively through participating EMA laboratories.
EMA First Step from Oral Arts
Don’t have time to make this device in your office? Simply send upper and lower models or impressions to Oral Arts Dental Lab. When your patient is ready to progress to the final EMA custom appliance, we will already have your modelwork!
- Centric bite registration
- Upper and lower impressions or models
- Patient friendly, metal-free design
- FDA approved and clinically proven to safely treat OSA and snoring
- Enhanced patient compliance in using the device on a nightly basis
- Lower cost than most other FDA approved oral appliances
- Can be used for any patient with either a full or partial set of natural teeth.
- Remaining teeth should have sufficient height of contour for the device to gain retention at the gingival third.
- Snoring
- Obstructive sleep apnea
- Edentulous patients
- Patients with braces
- Patients with dentures
- Used as a bleaching tray
- It can be fitted to a partial denture only if it has adequate retention and the patient sleeps with it in place.
- Cannot be used in cases of myofacial dysfunction, arthropathy of the temporomandibular joint and advanced periodontal problems.
- D5999 – Unspecified Maxillofacial Prosthesis, By Report. Used for procedure that is not adequately described by a code. Describe procedure.