SomnoDent® Sleep Apnea Device
FDA Cleared
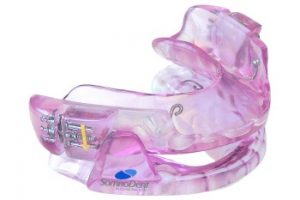
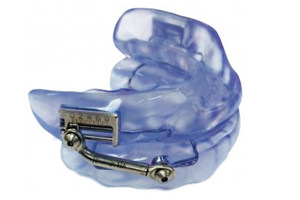
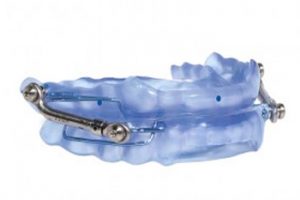
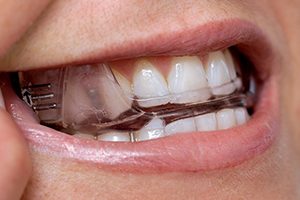
 Worn during sleep, SomnoDent® devices fit over the upper and lower teeth. The device slightly moves the mandible forward, helping to keep the airway open and allowing you to breathe normally. This movement tightens the soft tissue and muscles of the upper airway, which prevents obstructive apneas while you sleep. The SomnoDent® line of devices are customized for the patient and offer superior comfort and quality. Patients can talk and open/close their mouths and lips while wearing their SomnoDent sleep apnea device. SomnoDent devices are fabricated using the highest quality acrylic which does not discolor or attract odors.
Worn during sleep, SomnoDent® devices fit over the upper and lower teeth. The device slightly moves the mandible forward, helping to keep the airway open and allowing you to breathe normally. This movement tightens the soft tissue and muscles of the upper airway, which prevents obstructive apneas while you sleep. The SomnoDent® line of devices are customized for the patient and offer superior comfort and quality. Patients can talk and open/close their mouths and lips while wearing their SomnoDent sleep apnea device. SomnoDent devices are fabricated using the highest quality acrylic which does not discolor or attract odors.

Fusion
Custom Calibration
- Patient specific calibration
- 3 removable wings for 1mm of protrusion on the bottom
- Fine calibration in .1mm increments with lugs on the top
- Longer range of advancements (8.5mm) leading to fewer device resets
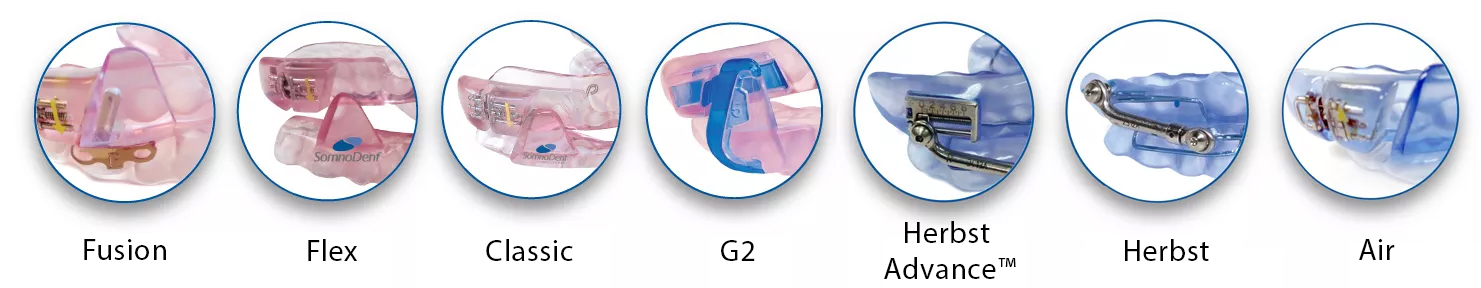
Flex
Premium Comfort
- Proprietary SMH BFlex inner liner for superior retention
- Drop in fit – even for patients with short teeth, little undercut or crown/bridge work
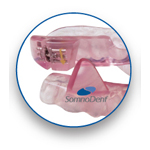
Classic
Original Innovation
- Highest quality acrylic
- Medical grade stainless steel ball clasps for retention
- Lingual-less option available for additional tongue space

G2
Latest Innovation
- Ease of calibration
- Wide range of calibration (-2mm to +8mm)
- Standard metal-free, also available with ball clasps
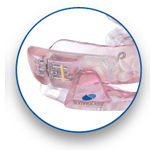
Herbst Advance™
Visual Simplicity
- Visual calibration indicator makes it easy to measure how far device has been advanced
- SMH BFlex offers superior comfort and retention
- Longer rance of advancement (8mm) leading to fewer device resets over the life of the device

Herbst®
Popular Value
- Standard telescopic mechanism (also available in Shim)
- SMH BFlex offers superior comfort and retention, also available with ball clasps
- Construction bite open 5 mm incisally that reflects a position of the mandible approximately 50-60% of maximum protrusive distance from centric occlusion to full protrusive.
- Upper and lower impressions or models
- See the Impression Guide for best results.
- The treatment of nighttime snoring and mild to moderate obstructive sleep apnea in patients 18 years of age or older.
The device is contraindicated for patients who:
- have central sleep apnea
- have severe respiratory disorders
- have loose teeth
- have advanced periodontal disease
- are under 18 years of age
≥ 14 Days
- D5999 – Unspecified Maxillofacial Prosthesis, By Report. Used for procedure that is not adequately described by a code. Describe procedure.
No warranty on SomnoDent appliances

Download and print an Oral Arts Sleep Apnea Flyer to use as an office display for your patients!